Patient Resources
Patient Resources
Resources to assist new and existing EyeSpace patients.
PDF Downloads

Bespoke Care and Maintenance 
Forge Ortho-K Care and Maintenance 
Scleral Care and Maintenance 
What are Bespoke Lenses 
What are Scleral Lenses 
What is Forge Ortho-K
Videos
Here are some videos
Glossary
View the medical device symbol glossary. Click on each term to view further details
Attention: Symbols on this page are for reference only. Products shown on the EyeSpace Lenses website may not be available in all markets and not all the symbols listed are applicable to your device.
 Manufacturer
Manufacturer
Explanation
Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC
Standard Title
Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirement
Standard Reference
- ISO 15223-1:2016 Ref no: 5.1.1
- FDA Recognition no: 5-117
- ISO 7000 Ref no: 3082
- FDA Recognition no 5-124
- EN 980, Clause 5.12
 Batch Code
Batch Code
Explanation
Indicates the manufacturer’s batch code so that the batch or lot can be identified.
Standard Title
Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirement
Standard Reference
- ISO 15223-1:2016 Ref no: 5.1.5
- FDA Recognition no: 5-117
- ISO 7000 Ref no: 2492
- FDA Recognition no 5-124
- EN 980, Clause 5.4
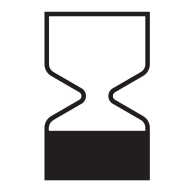 Use-by Date
Use-by Date
Explanation
Indicates the date after which the medical device is not to be used.
Standard Title
Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements
Standard Reference
- ISO 15223-1:2016 Ref no: 5.1.4
- FDA Recognition no: 5-117
- ISO 7000 Ref no: 2607
- FDA Recognition no 5-124
- EN 980, Clause 5.3
 Consult instructions before use
Consult instructions before use
Explanation
Indicates the need for the user to consult the instructions for use
Standard Title
Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements
Standard Reference
- ISO 15223-1:2016 Ref no: 5.4.3
- FDA Recognition no: 5-117
- ISO 7000 Ref no: 1641
- FDA Recognition no: 5-124
- EN 980, Clause 5.18
 QR Code
QR Code
Explanation
Directs user to Patient Resources
Standard Title
Information technology — Automatic identification and data capture techniques — QR Code 2005 bar code symbology specification
Standard Reference
- ISO/IEC 18004:2006
 Recyclable
Recyclable
Explanation
Indicates the packaging is recyclable
Standard Title
Graphical symbols for use on equipment — Registered symbols
Standard Reference
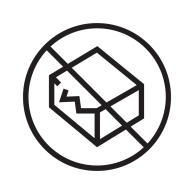 Do not use if package information is damaged
Do not use if package information is damaged
Explanation
Indicates a medical device that should not be used if the package has been damaged or opened
Standard Title
Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements
Standard Reference
- ISO 15223-1 Ref no: 5.2.8
- FDA Recognition no 5-117
- ISO 7000 Ref no: 2606
- FDA Recognition no: 5-124
 Prescription Only
Prescription Only
Explanation
Caution: Federal law restricts this device to sale by or on the order of a contact lens prescriber
Standard Title
Labeling-Prescription devices. Indicates the product is a medical device as defined in 21 CFR 820.3(l) and contact lens prescriber as defined in 16 CFR 315.2
Standard Reference
- 21 CFR 801.109
- 21 CFR 801.15(c)(1)(i)F